A century has passed since the discovery of the Warburg Effect, a metabolic hallmark of cancer cells that remains central to understanding and treating cancer. Despite extensive research and significant financial investments, cancer continues to defy complete understanding and effective treatment. Otto Warburg’s groundbreaking observation reshaped our understanding of cancer metabolism and remains a cornerstone of therapeutic innovation.
The Warburg Effect
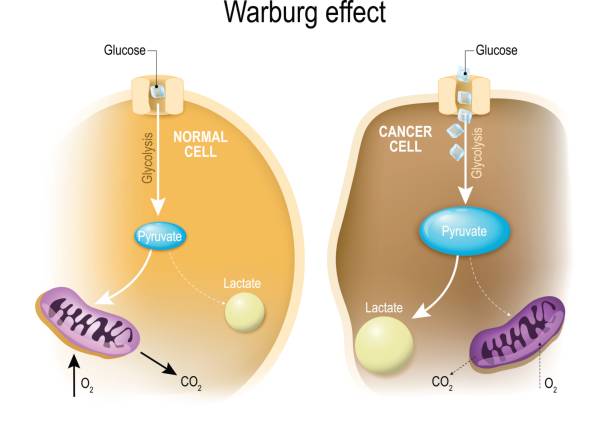
In 1924, Otto Warburg, while studying a rat seminal vesicle tumor observed that rapidly dividing cells consumed large amounts of glucose and excreted lactate, despite reduced oxygen usage. Under normal conditions, cells use mitochondria to oxidize glucose in the presence of oxygen, but in oxygen-deprived conditions, glucose is converted into lactate. Warburg discovered that cancer cells uniquely consumed elevated levels of glucose even in oxygen-rich environments, accompanied by increased lactate production. This phenomenon, known as aerobic glycolysis or the Warburg Effect, is now recognized across numerous tumor types, highlighting a critical feature of cancer cell metabolism.
The Economics of Energy Metabolism
Cancer cells rely on the Warburg Effect not just for energy generation but also to meet their diverse metabolic needs. To understand its significance, it is essential to consider how cells generate energy:
Adenosine Triphosphate (ATP) is the primary energy currency of mammalian cells, produced via two pathways:
- Oxidative Phosphorylation: Generates up to 36 ATP molecules per glucose molecule in oxygen-rich conditions.
- Glycolysis(Non-oxidative): Produces only 2 ATP molecules per glucose molecule but does so rapidly. Conventional cellular energy models suggest that oxygen availability dictates the most efficient ATP-generating pathway, with glycolysis acting as a backup system during oxygen-deprived (hypoxic) conditions.
- While most cells prioritize oxidative phosphorylation for efficiency, cancer cells defy this logic by favoring glycolysis even in sufficient oxygen resulting in a compensatory increased glucose uptake to sustain their metabolic demands.
Mechanisms Driving the Warburg Effect
The Warburg Effect is sustained through profound reprogramming of metabolic pathways in cancer cells, driven by genetic and molecular changes. Key Molecular Drivers of the Warburg Effect:
1. The HIF-1, c-MYC, and mTOR Axis
- HIF-1 (Hypoxia-Inducible Factor-1): Promotes the production of glycolytic enzyme under low oxygen.
- c-MYC: Enhances the expression of genes that support glycolysis and anabolic pathways.
- mTOR: Stimulates protein synthesis and metabolic reprogramming in response to growth signals.
In normal cells, HIF-1 suppresses c-MYC under low-oxygen conditions. However, in cancer cells, these pathways work together to enhance glycolysis. They increase the expression of glucose transporters (GLUTs), and key enzymes like pyruvate kinase M2 (PKM2). PKM2 not only fuels glycolysis but also diverts glucose intermediates to biosynthetic pathways critical for tumor growth.
2. Loss of p53 Function
p53, a key tumor suppressor, plays a central role in regulating cellular metabolism:
- In Normal Cells: p53 inhibits glycolysis by repressing glucose transporters and activating the tumor-suppressive PTEN pathway, which counteracts PI3K/Akt signaling.
- In Cancer Cells: Loss of p53 removes these inhibitory controls, allowing glycolysis to proceed unchecked. This metabolic shift supports the high energy and biosynthetic demands of cancer cells, reinforcing the Warburg Effect.
Benefits of the Warburg Effect for Cancer Cells
The Warburg Effect offer several distinct advantages that support tumor growth and survival:
- Rapid ATP Production
- Glycolysis, though less efficient than oxidative phosphorylation, delivers ATP at a faster rate. This ensures an immediate energy supply for rapidly dividing cancer cells.
- Lactate production regenerates NAD+(Nicotinamide Adenine Dinucleotide), sustaining continuous glucose metabolism.
- Support for Biosynthesis
Beyond energy, rapid glucose metabolism provides essential building blocks for cancer cell growth. These include:
- Nucleotides for DNA replication.
- Amino acids for protein synthesis.
- Lipids for the formation of cell and organelle membranes.
- Folic acid to support DNA synthesis.
- NAD for facilitating biochemical reactions.
These resources enable the synthesis of components required for rapid cell division and growth.
- Creation of an Acidic Microenvironment
Cancer cells release excess lactate, acidifying their surrounding.
This acidic environment:
- Promote tumor invasion and metastasis(spread).
- Weakens immune cell activity, allowing tumors to evade immune surveillance
The Warburg Effect, therefore, plays a critical role in helping cancer cells meet their energy needs, sustain growth, and create conditions favorable for tumor progression.
Host Impact: Consequences of the Warburg Effect
The metabolic reprogramming in cancer cells has significant systemic and local effects on the host:
•Nutrient Depletion: Cancer cells consume large amounts of glucose, depriving normal cells and contributing to conditions like cachexia—a severe wasting syndrome.
•Acidic Microenvironment: This acidic environment can damage surrounding tissues, disrupt normal cellular activities, and further contribute to the progression of cancer.
•Resource Competition: Cancer cells outcompete normal cells for glucose and other nutrients, leading to tissue deterioration and worsening of the overall health of the host.
•Organ Dysfunction: Metabolic byproducts accumulate and impair vital organs, particularly the liver and kidneys, accelerating organ failure and further weakening of the host.
Therapeutic Implications: Targeting the Warburg Effect
The Warburg Effect offers promising opportunities for therapeutic interventions by exploiting cancer cells’ metabolic vulnerabilities.
Inhibiting Glycolysis: Starving Cancer Cells
- Glycolysis Blockers: Drugs like 2-deoxyglucose (2-DG) mimic glucose, disrupting its metabolism and starving cancer cells.
- Glucose Transport Inhibitors: Drugs like Phloretin and WZB117 block glucose transporters, which help cancer cells absorb glucose, thereby reducing glucose uptake.
Targeting Key Glycolytic Enzymes
- Hexokinase Inhibitors: Drugs like Lonidamine and 3-bromopyruvate (3BP) block hexokinase, reducing glycolysis and triggering energy starvation in cancer cells.
- Lactate Dehydrogenase (LDH) Inhibitors: LDH converts pyruvate to lactate. Blocking LDH reduces lactate production, weakening cancer cells and their acidic environment.
Targeting Lactate and Acidic Tumor Environment
- MCT Inhibitors: Cancer cells use monocarboxylate transporters (MCTs) to remove lactate. Drugs like AZD3965 block MCTs, causing lactate buildup, acidifying cancer cells internally, and reducing tumor microenvironment acidity.
- Improved Immune Response: Lower acidity enhances immune therapies like checkpoint inhibitors, making them more effective.
Exploiting Tumor Hypoxia
- HIF-1 Inhibitors: Hypoxia-inducible factor-1 (HIF-1) helps cancer cells adapt to low oxygen. Drugs like Acriflavine disrupt HIF-1, forcing cancer cells to rely on oxidative metabolism, which they struggle to use.
- Hypoxia-Activated Drugs: Prodrugs like Tirapazamine are activated in low-oxygen areas, targeting hypoxic cancer cells while sparing healthy tissues.
Combining Therapies for Better Results
- Glycolysis Inhibitors and Chemotherapy: Drugs like 2-DG paired with chemotherapy enhance cancer cell death.
- Improved Immunotherapy: Reducing lactate or acidity can boost immune cells’ ability to fight cancer when combined with treatments like immune checkpoint inhibitors.
Dietary Approaches to Target Cancer Metabolism
- Ketogenic Diet: A low-carb, high-fat diet deprives cancer cells of glucose, their primary energy source, slowing tumor growth. Ongoing clinical trials are evaluating its potential.
- Intermittent Fasting: Temporarily lowering glucose through fasting can stress cancer cells and improve chemotherapy effectiveness.
By targeting the Warburg effect, therapies can weaken cancer cells’ metabolic advantages, enhance existing treatments, and improve patient outcomes. These approaches exploit cancer’s dependence on glycolysis and lactate production, offering promising avenues for personalized cancer care.
Challenges and Future Directions
While targeting the Warburg Effect holds great promise, several challenges remain:
- Side Effects on Normal Cells: Glycolysis is essential for many normal tissues, especially red blood cells, which rely entirely on glycolysis for energy. Additionally, many glycolytic enzymes perform critical roles beyond metabolism, and inhibiting them could disrupt these processes, leading to unintended side effects in healthy tissues.
- Tumor Heterogeneity: Not all tumors depend on glycolysis to the same extent. This variability necessitate personalized approaches.
- Cancer’s Adaptability: Cancer cells are highly adaptable and can switch to alternative energy pathways, such as oxidative phosphorylation when glycolysis is inhibited. This flexibility complicates efforts to disrupt their metabolism effectively.
Future Directions
- Selective Inhibitors: Develop inhibitors that target only cancer-specific metabolic pathways, minimizing harm to normal tissues.
- Combination Therapies: Combine metabolic therapies with immunotherapy, radiation, or chemotherapy to achieve synergistic effects and improve treatment efficacy.
- Advancing Research with AI: Leverage Artificial intelligence(AI) and biomarker discovery to deepen our understanding of cancer metabolism and identify new therapeutic targets.
Conclusion: Toward a Future of Metabolic Therapy
The Warburg effect reveals the unique metabolic strategies cancer cells use to sustain their growth, providing valuable opportunities for therapeutic intervention. As research progresses, more treatments designed to exploit these metabolic weaknesses are likely to emerge.
However, cancer’s adaptability remains a significant challenge. Combining metabolic therapies with treatments like immunotherapy, radiation, or chemotherapy will likely yield the most durable result.
The Warburg effect extends beyond energy production—it’s how cancer cells manipulate their environment to outcompete healthy cells and hijack the body’s resources. By targeting these vulnerabilities, we can weaken cancer while restoring the body’s natural balance.
As our understanding of cancer metabolism grows, new tools and strategies will bring us closer to a future where cancer is more treatable and survival rates continue to improve.
Further Readings
Epstein T, Gatenby RA, Brown JS (2017) .The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand.
Liberti MV, Locasale JW(2016). The Warburg Effect: How Does it Benefit Cancer Cells?
Barba I, Carrillo-Bosch L, Seoane J(2024). Targeting the Warburg Effect in Cancer: Where Do We Stand?
Tran Q, Lee H, Kim C, Kong G, Gong N, Kwon SH, Park J, Kim SH, Park J(2020). Revisiting the Warburg Effect: Diet-Based Strategies for Cancer Prevention.